4.1 describe the use of the indicators litmus, phenolphthalein and methyl orange to distinguish between acidic and alkaline solutions
Litmus paper (comes in either blue or red)
Acidic: red
Alkaline: blue
Phenolphthalein
Acidic: colourless
Alkaline: pink
Methyl orange
Acidic: Yellow
Alkaline: Red
4.2 understand how the pH scale, from 0–14, can be used to classify solutions as strongly acidic, weakly acidic, neutral, weakly alkaline or strongly alkaline
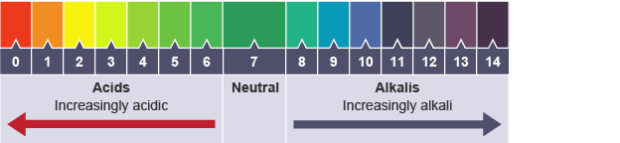
pH 0 – 3 : strongly acidic
pH 4 – 6: weakly acidic
pH 7: neutral
pH 8 – 10: weakly alkaline
pH 11 – 14: strongly alkaline
4.3 describe the use of universal indicator to measure the approximate pH value of a solution

Add universal indicator to solution. It will change colour depending on its pH.
4.4 define acids as sources of hydrogen ions, H+, and alkalis as sources of hydroxide ions, OH ̄
Acids are a source of hydrogen ions (H+).
Alkalis are a source of hydroxide ions ( OH-)
For example, if hydrogen chloride gas is dissolved in water, the hydrogen and chlorine ions dissasociate.
HCl –> H+ + Cl-
If NaOH is dissolved in water, the sodium and hydroxide ions dissasociate.
NaOH –> Na+ + OH-
4.5 predict the products of reactions between dilute hydrochloric, nitric and sulfuric acids; and metals, metal oxides and metal carbonates (excluding the reactions between nitric acid and metals)
hydrochloric acid: HCl
sulfuric acid: H₂SO₄
nitric acid: HNO3
Acids and metals
Acid + metal –> Salt + Hydrogenhydrochloric acid + metal–> metal chloride salt + hydrogen
sulfuric acid + metal –> metal sulfate salt + hydrogen
sulfuric acid + metal –> metal sulfate salt + hydrogen
Acids and metal oxides
Acid + metal oxide –> Salt + Water
hydrochloric acid + metal oxide–> metal chloride + water
sulfuric acid + metal oxide –> metal sulfate salt + water
nitric acid + metal oxide–> metal nitrate salt + water
Acids and metal hydroxides
Acid + metal oxide –> Salt + Water
hydrochloric acid + metal hydroxide–> metal chloride + water
sulfuric acid + metal hydroxide–> metal sulfate salt + water
nitric acid + metal hydroxide–> metal nitrate salt + water
Acids and metal carbonates
Acid + metal carbonate –> Salt + Water + Carbon Dioxide
hydrochloric acid + metal carbonate–> metal chloride + water + carbon dioxide
sulfuric acid + metal carbonate–> metal sulfate salt + water + carbon dioxide
nitric acid + metal carbonate–> metal nitrate salt + water + carbon dioxide
4.6 understand the general rules for predicting the solubility of salts in water:
i all common sodium, potassium and ammonium salts are soluble
ii all nitrates are soluble
iii common chlorides are soluble, except silver chloride
iv common sulfates are soluble, except those of barium and calcium
v common carbonates are insoluble, except those of sodium, potassium and ammonium
| Soluble | Insoluble |
|---|---|
| All nitrates | None |
| Most sulfates | Lead sulfate, barium sulfate and calcium sulfate |
| Most chlorides, bromides and iodides | Silver chloride, silver bromide, silver iodide, lead chloride, lead bromide, lead iodide |
| Sodium carbonate, potassium carbonate, ammonium carbonate | Most other carbonates |
| Sodium hydroxide, potassium hydroxide, ammonium hydroxide | Most other hydroxides |
4.7 describe experiments to prepare soluble salts from acids
- Add metal/metal oxide/metal hydroxide/metal carbonate to acid until it no longer dissolves (this means it is in excess)
- Filture solution to get rid of excess
- Pour filtered solution into evaporation basin
- Heat it using a bunsen burner until half of the solution has been evaporated off
- Allow to cool
- Filter again
- Pat crystals dry with paper towels
4.8 describe experiments to prepare insoluble salts using precipitation reactions
- Mix two solutions together (both from soluble salts)
- A precipitate will form
- Filter the mixture
- Wash the precipate with water
- Leave to dry
4.9 describe experiments to carry out acid-alkali titrations.

- Fill a burette with a known volume of acid
- Fill 25cm^3 glass pipette with alkali and put that alkali substance in a conical flask
- Add 3 drops of indicator (phenolphthalein/methyl orange) to the alkali
- Drip the acid from the burette into the conical flask with the alkali using the tap. Mix constantly while doing so
- Stop when colour change occurs
- Repeat the process using the known volume of acid and known volume of alkali without the use of the indicatior
- This is a neutral solution
